Theraputic Enzymes
Engineering Enzymes Hydrolyzing Quorum Sensing Molecules
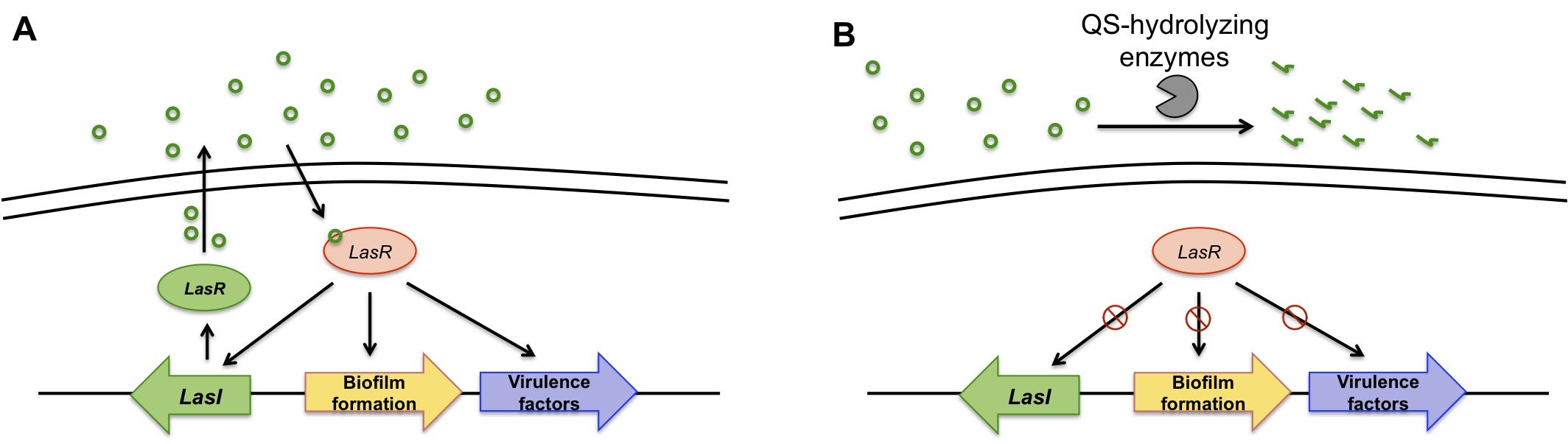
Bacterial biofilms are responsible for persistent infections inducing cystic fibrosis, endocarditis, chronic wounds, etc, which impact tens of millions of Americans each year. Mounting evidence implicated that the expression of virulence factors by the pathogenic bacteria is controlled through intercellular communication mediated by quorum sensing (QS). Therefore, QS molecules, e.g. N-acyl homoserine lactones and autoinducers, have been recognized as very attractive pharmaceutical targets for fighting antibiotics resistance. Unlike antimicrobials that mimic QS molecules for QS interfering, enzymatic hydrolysis/degradation of QS molecules cause less selection pressure for bacterial resistance. Taking the advantages of high activity, easy for expression and amenable to engineering, bacterial lactonases have been extensively studied. However, clinic applications of bacterium-based enzymes are largely hampered due to their immunogenicity. We apply rational and combinatorial approaches to improve the activity of a novel class of lactone-hydrolyzing enzymes based on human proteins to address this immunogenicity issue. These engineered enzymes can also be formulated for stimuli triggered delivery and for evaluation on the treatment efficacy in mouse models. Collaborators: Dr. Ashok Mulchandani (UCR); Dr. Manuela Martins-Green (UCR). Funding: UCR Seed Grant.