Protease Inhibitory Antibodies
Protease Inhibitory Antibodies
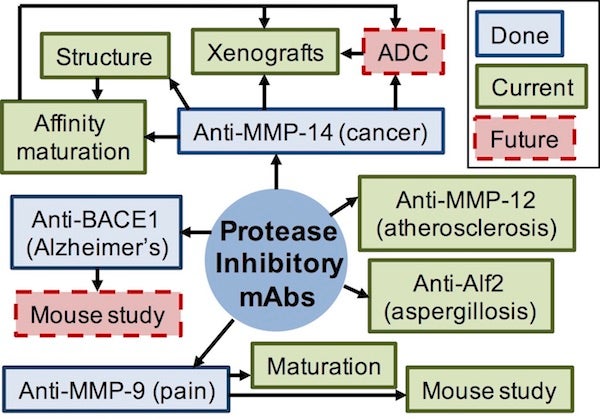
As extremely important signaling molecules, proteases precisely control a wide variety of physiological processes, and thus represent one of the largest families of potential pharmaceutical targets. Mounting evidence taught us that specificity is desired for any protease inhibition therapy. However, the proteolytic mechanisms are highly conserved among the same class or family of proteases, thus distinguishing proteases using small molecule inhibitors is exceedingly difficult (e.g. fails of board spectrum MMP inhibitors in clinical trials). Because of their exquisite specificity, antibody-based inhibitors are emerging as promising protease-blocking agents. Unfortunately, to date, at least two major obstacles make the routine discovery of protease-inhibiting mAbs difficult: (1) low antigenicity of protease active sites, and (2) lack of function-based selection methods. Our long-term goal is to develop therapeutic mAbs that would inhibit specific proteases in disease. The short-term objectives are to (1) overcome these obstacles and establish general, streamlined methodologies for the discovery and engineering of inhibitory antibodies; (2) test therapeutic potentials of isolated mAbs in xenograft models of relative diseases.
By designing and constructing novel synthetic antibody library (PNAS 2016), and establishing functional selection methods based on genetic selection (ongoing), dual color FACS (PEDS 2017) and deep DNA sequencing (B&B 2017), we isolated a panel of highly potent and selective Fab inhibitors targeting all four main classes of proteases: matrix metalloproteinases (MMP-14, a predominant target in cancer metastasis; MMP-9, in neuropathic pains), beta-secretase 1 (BACE1, an aspartic protease in Alzheimer), cathepsin K (a cysteine protease in cancer), and Alp2 (a serine protease in aspergillosis). Particularly, Fab 3A2 blocks MMP-14 hydrolyzing its physiological substrates with a binding affinity of 4.8 nM and an inhibition potency of 9.7 nM. Enzyme inhibition kinetics, competitive ELISA and epitope mapping studies collectively suggested that Fab 3A2 was a competitive inhibitor binding to the vicinity of MMP-14 reaction cleft (PNAS 2016). Further, in melanoma mouse model, mAb 3A2 significantly reduced cancer cell spreading (Oncotarget 2017). In addition, intravenous application of mAb L13, an anti-MMP9 inhibitor isolated by genetic selection with a 20 nM potency, exhibited pain attenuation effects in paclitaxel mouse model (ongoing). Overall, highly potent mAb inhibitors with exclusive selectivity have been discovered for many proteases of biomedical importance with the potentials to provide desired therapeutic efficacy.