Publications
Book Chapters
2. Nam DH, Ge X. Generation of Highly Selective MMP Antibody Inhibitors. In Proteases and Cancer. Methods in Molecular Biology (Clifton, N.J.) 1731:307-324 Jan 2018.
1. Nam DH, Lee KB, Ge X. Functional production of catalytic domains of human MMPs. In Proteases and Cancer. Methods in Molecular Biology (Clifton, N.J.) 1731: 65-72 Jan 2018.
Journal Articles
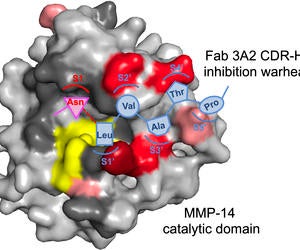
38. Nam DH, Lee KB, Kruchowy E, Pham H, Ge X. Protease inhibition mechanism of camelid-like synthetic human antibodies. Biochemistry (2020) 59[40]:3802-3812. Link
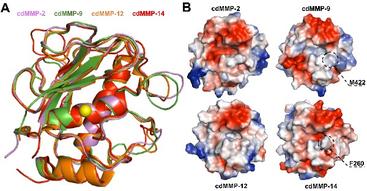
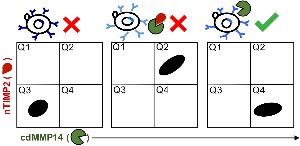
37. Lee KB, Dunn Z, Lopez T, Mustafa Z, Ge X. Generation of highly selective monoclonal antibodies inhibiting a recalcitrant protease using decoy designs. Biotechnology and Bioengineering (2020) 117[12]:3664-3676. Link

36. Li X, Zhao Y, Chen C, Lee H-H, Wang Z, Zhang N, Kolonin MG, An Z, Ge X, Scherer PE, Sun K. The critical role of MMP14 in adipose tissue remodeling during obesity. Molecular and Cellular Biology (2020) 40[8]:e00564-19.
35. Mei M, Li J, Wang S, Lee KB, Iverson BL, Zhang G, Ge X, Yi L. Prompting Fab Yeast Surface Display Efficiency by ER Retention and Molecular Chaperon Coexpression. Frontiers in Bioengineering and Biotechnology (2019) 7:362.
34. Sedki M, Chen X, Chen C, Ge X, Mulchandani A. Non-lytic M13 Phage-based Highly Sensitive Impedimetric Cytosensor for Detection of Coliforms. Biosensors and Bioelectronics (2020) 148:111794.
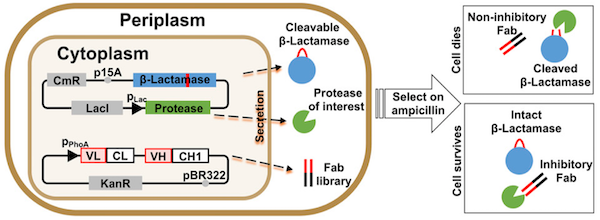
33. Lopez T, Mustafa Z, Chen C, Lee KB, Ramirez A, Benitez C, Luo X, Ji R-R, Ge X. Functional Selection of Protease Inhibitory Antibodies. Proceedings of the National Academy of Sciences of the United States of America (2019) 116[33]:16314-16319.
32. Yu X, Niks D, Ge X, Liu H, Hille R, Mulchandani A. Synthesis of Formate from CO2 Gas Catalyzed by an O2-tolerant NAD-Dependent Formate Dehydrogenase and Glucose Dehydrogenase. Biochemistry (2019) 58[14]:1861-1868. Link
31. Lopez T, Ramirez A, Benitez C, Mustafa Z, Pham H, Sanchez R, Ge X. Selectivity Conversion of Protease Inhibitory Antibodies. Antibody Therapeutics (2018) 1[2]:55-63.
30. Lee KB, Dunn Z, Ge X. Reducing proteolytic liability of a MMP‐14 inhibitory antibody by site‐saturation mutagenesis. Protein Sciences (2018) 28[3]:643-653. Link
29. Lopez T, Chen C, Ramirez A, Chen KE, Lorenson MY, Benitez C, Mustafa Z, Pham H, Sanchez R, Walker AM, Ge X. Epitope Specific Affinity Maturation Improved Stability of Potent Protease Inhibitory Antibodies. Biotechnology and Bioengineering (2018) 115[11]:2673-2682.
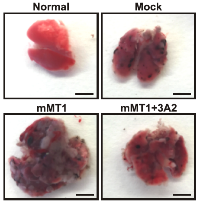
28. Chen KE, Chen C, Radecki KC, Bustamante K, Lopez T, Lorenson MY, Ge X, Walker AM. Use of a Novel Camelid-inspired Human Antibody Demonstrates the Importance of MMP-14 to Cancer Stem Cell Function in the Metastatic Process. Oncotarget (2018) 9[50]:29431-29444.
27. Wang J, Boddupalli A, Koelbl J, Nam DH, Ge X, Bratlie K, Schneider IC. Degradation and Remodeling of Epitaxially Grown Collagen Fibrils. Cellular and Molecular Bioengineering (2018) doi.org/10.1007/s12195-018-0547-6. Link
26. Chen L, Ge X. Correlation Between Size and Activity Enhancement of Recombinantly Assembled Cellulosomes. Applied Biochemistry and Biotechnology (2018) 186[4]:937-948. Link
25. Limsakul P, Peng Q, Wu Y, Allen M, Liang J, Remacle AG, Lopez T, Ge X, Kay B, Zhao H, Strongin AY, Yang X-L, Lu S, Wang Y. Directed Evolution to Engineer Monobody for FRET Biosensor Assembly and Imaging at Live-Cell Surface. Cell Chemical Biology (2018) 25[4]:370-379, Link
24. Chen L, Holmes M, Schaefer E, Mulchandani A, Ge X. Highly Active Spore Biocatalyst by Self-Assembly of Co-Expressed Anchoring Scaffoldin and Multimeric Enzyme. Biotechnology and Bioengineering (2018) 115[3]:557-564.
23. Rodriguez C, Nam DH, Kruchowy E, Ge X. Efficient Antibody Assembly in E. coli Periplasm by Chaperone Co-expression and Culture Optimization. Applied Biochemistry and Biotechnology (2017) 183[2]:520-529.
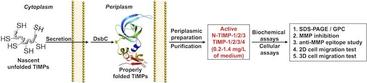
22. Lee KB, Nam DH, Nuhn JA, Wang J, Schneider IC, Ge X. Direct expression of active human tissue inhibitors of metalloproteinases by periplasmic secretion in Escherichia coli. Microbial Cell Factories (2017) 16[1]:73.
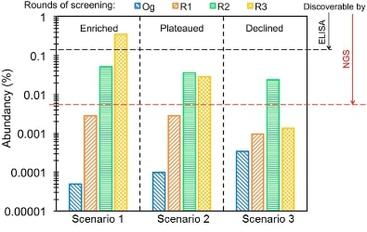
21. Lopez T, Nam DH, Kaihara E, Mustafa Z, Ge X. Identification of Highly Selective MMP-14 Inhibitory Fabs by Deep Sequencing. Biotechnology and Bioengineering, (2017) 114[6]:1140-1150.
20. Nam DH, Fang K, Rodriguez C, Lopez T, Ge X. Generation of inhibitory monoclonal antibodies targeting membrane-type 1 matrix metalloproteinase by motif grafting and CDR optimization. Protein Engineering Design and Selection (2017) 30[2]:113-118.
19. Chen L, Mulchandani A, Ge X. Spore-Displayed Enzyme Cascade with Tunable Stoichiometry. Biotechnology Progress(2016), 33[2]: 383-389.
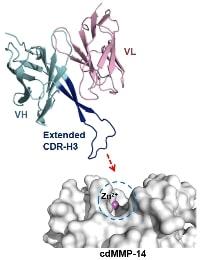
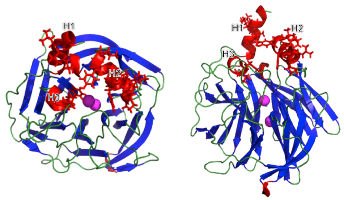
18. Nam DH, Rodriguez C, Remacle AG, Strongin AY, Ge X. Active-site MMP-selective antibody inhibitors discovered from convex paratope synthetic libraries. Proc Natl Acad Sci USA (2016), 113[52]:14970-14975.
Reported by 12 media and UCR Today: AAAS Eurekalert, Genetic Engineering & Biotechnology News (GEN),Medical News Today, Medical Xpress, Health Canal, Drug Discovery and Development, Business Standard, Today Topics, Technology.org, NEWS.am, Fierce Biotech, Medindia.
17. Remacle AG, Cieplak P, Nam DH, Shiryaev SA, Ge X, Strongin AY. Selective Function-Blocking Monoclonal Human Antibody Highlights the Important Role of Membrane Type-1 Matrix Metalloproteinase (MT1-MMP) in Metastasis. Oncotarget(2017) 8:2781-2799.
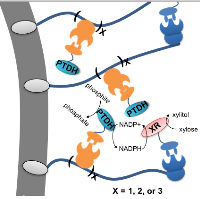
16. Li XC, Wang C, Mulchandani A, Ge X. Engineering Soluble Human Paraoxonase 2 for Quorum Quenching. ACS Chemical Biology (2016) 11[11]: 3122-3131.
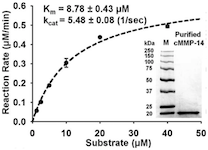
15. Nam DH, Ge X. Direct Production of Functional Matrix Metalloproteinase -14 Without Refolding or Activation and its Application for In Vitro Inhibition Assays. Biotechnology and Bioengineering (2016) 113[4]: 717-723.
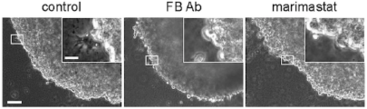
14. Haage A, Nam DH, Ge X, Schneider I. Matrix Metalloproteinase-14 is a Mechanically Regulated Activator of Secreted MMPs and Invasion. Biochemical and Biophysical Research Communications. (2014) 450[1]: 213-218.
13. Nam DH, Ge X. Development of Periplasmic FRET-Based Screening Method for Protease Inhibitory Antibodies. Bioengineering and Biotechnology. (2013) 110[11]: 2856-2864.
12. Eleftheriou NM, Ge X, Kolesnik J, Falconer SB, Harris RJ, Khursigara C, Brown ED, Brennan JD. Entrapment of Living Bacterial Cells in Low-Concentration Silica Materials Preserves Cell Division and Promoter Regulation. Chemistry of Materials. (2013) 25[23]: 4798-4805.
11. Ge X, Eleftheriou NM, Dahoumane SA, Brennan JD. Sol-Gel Derived Materials for Production of Pin-Printed Reporter Gene Living-Cell Microarrays. Analytical Chemistry (2013) 85[24]: 12108-12117.
10. Ippolito GC, Hoi KH, Reddy ST, Carroll SM, Ge X, Rogosch T, Zemlin M, Shultz LD, Ellington AD, Vandenberg CL, Georgiou G. Antibody Repertoires in Humanized NOD-scid-IL2Rγ(null) Mice and Human B Cells Reveals Human-Like Diversification and Tolerance Checkpoints in the Mouse. PLoS One (2012) 7[4]: e35497.
9. Ge X, Lebert JM, Monton MRN, Lautens LL, Brennan JD. Materials Screening for Sol–Gel-Derived High-Density Multi-Kinase Microarrays. Chemistry of Materials (2011) 23[16]: 3685-3691.
8. Ge X, Mazor Y, Hunicke-Smith SP, Ellington AD, Georgiou G. Rapid Construction and Characterization of Synthetic Antibody Libraries Without DNA Amplification. Biotechnology and Bioengineering (2010) 106[3]: 347-357.
7. Reddy ST, Ge X, Miklos AE, Hughes RA, Kang SH, Hoi KH, Chrysostomou C, Hunicke-Smith SP, Iverson BL, Tucker PW, Ellington AD, Georgiou G. Monoclonal Antibodies Isolated Without Screening by Analyzing the Variable-Gene Repertoire of Plasma Cells. Nature Biotechnology (2010) 28[9]: 965-969.
Featured by Cell (2010) 143:491. "Better Antibodies Through Sequencing" - Biotechniques News 10/5/2010.
6. Ge X, Hoare T, Filipe CDM. Protein Based Aqueous-Multiphasic Systems. Langmuir (2010) 26[6]: 4087-4094.
5. Ge X, Conley A, Brandle JE, Truant R, Filipe CDM. In Vivo Formation of Protein Based Aqueous Microcompartments. Journal of the American Chemical Society (2009) 131[25]: 9094-9099.
4. Ge X, Trabbic-Carlson K, Chilkoti A, Filipe CDM. Purification of an Elastin Like Fusion Protein by Microfiltration.Biotechnology and Bioengineering (2006) 95[3]: 424-432.
3. Ge X, Filipe CDM. Simultaneous Phase Transition of ELP Tagged Molecules and Free ELP: An Efficient and Reversible Capture System. Biomacromolecules (2006) 7[9]: 2475-2478.
2. Ge X, Yang DSC, Trabbic-Carlson K, Kim B, Chilkoti A, Filipe CDM. Self-Cleavable Stimulus Responsive Tags for Protein Purification Without Chromatography. Journal of the American Chemical Society (2005) 127[32]: 11228-11229.
Featured by Chemical & Engineering News (2005) 83[32]: 36. The Scientist (2005) 19[22]: 24. Faculty of 1000 Biology 16089436.
1. Ge X, Li Q. Humoral Immune Responses to Human Cardiac Troponin T by Genetic Immunization, Chinese Journal of Microbiology and Immunology. (2003) 23[7]: 544-547.
Patents
6. Lopez T, Ge X, Ji R-R. Highly Specific MMP-9 Inhibitory Monoclonal Antibodies for Neuropathic Pain Relief. US62/850,914 (2019-05-21 Application); UC Case No. 2019-764-1.
5. Lopez T, Ge X. Highly Specific MMP-9 Inhibitory Antibodies. US62/851,001 (2019-05-21, Application); UC Case No. 2019-118-1.
Licensed to Maverick Therapeutics, December 2018.
4. Nam DH, Ge X. Inhibitory Antibodies from Synthetic Long CDR Libraries. WO2018067198A1 (2018-04-12 Application); PCT/US2017/022341 (2017-03-14 Application); US16/223,943 (2018-12-18 Application); US-2019-0185581 (2019-06-20 Publication); UC Case No. 2015-758-3.
3. Reddy ST, Ge X, Boutz D, Ellington AD, Marcotte EM, Georgiou G. Rapid Isolation of Monoclonal Antibodies from Animals. US9090674B2 (2015-07-28Grant); EP2572203B1 (2017-10-25 Grant); KR20130135028A (2013-12-10Application); CN103003697A (2013-03-27 Application); CA2799746A1 (2011-11-24Application); WO2011146514A3 (2012-11-22 Application).
Licensed to Cell Signaling Technology Inc, May 2014.
2. Ge X, Mazor Y, Georgiou G. Methods for Creating Antibody Libraries. US12868399 (2010-08-25Pending); EP2470653A1 (2012-07-04 Application); CA2772298A1 (2011-03-03Application); WO2011025826A1 (2011-03-03 Application).
1. Chilkoti A, Carlson KT, Christensen T, Filipe CDM, Ge X. Purification of Low-Abundant Recombinant Proteins from Cell Culture. WO2008024311A3(2008-10-30 Application).
Licensed to PhaseBio Pharmaceuticals Inc, March 2008.